Who can believe that it’s already time to start thinking about 2021? Many may welcome the thought of putting 2020 in the past and it is important for pharmacists to start looking at how the CMS proposed changes for 2021 might impact collaborative clinical services. With the challenges of COVID-19, we’ve seen many interim changes to the rules and regulations, but it is difficult to know if some of these changes will be adopted permanently. We look to the proposed changes as guidance for the upcoming year and share the updates with you, keeping in mind that rules will be finalized towards the end of this year.
This is a big one! With more pharmacists and other healthcare professionals pursuing RPM services during COVID-19, there has been a lot of buzz on the rules and requirements of RPM. CMS acknowledged the ambiguity surrounding RPM services and clarified many areas of question as part of the proposed changes for 2021. Keep in mind that not all of these clarifications are being positively accepted by many currently offering RPM services. For those who are not familiar with Remote Physiologic Monitoring (RPM), commonly referred to as remote patient monitoring, it involves the collection and analysis of digitally collected physiologic data that is used to develop and guide treatment plans. CMS clarified that RPM services are appropriate for both chronic and acute illnesses or conditions.
In the proposed changes, CMS noted that 99453 for the initial set-up and patient education on use of equipment is intended to be billed once per episode of care. They defined an episode of care as “beginning when the remote physiologic monitoring service is initiated and ends with attainment of targeted treatment goals.” This code covers the time dedicated to educating a patient on the use of one or more medical devices. From this definition, it may be assumed that the code may be billed more than once if a patient must be initiated on a new device when a new treatment need is identified. Further clarification may be needed from the final rule as many had interpreted that this code was billable only once. CMS also clarified that since the CPT code descriptors do not specify that clinical staff must be the ones furnishing CPT codes 99453 and 99454, they can be completed by auxiliary personnel who are not considered to be clinical staff. As another update, policies now allow patient consent for RPM to be obtained at the time services are furnished and they even stated that consent can be obtained by individuals performing RPM services under contract with the billing physician or other practitioner. During the PHE of COVID-19, RPM services can be offered to both new and established patients of a practice but at the end of the PHE, patients must be established to be eligible for the service.
One of the areas of question for RPM was relevant to the device code, CPT code 99454. The CPT prefatory language states that monitoring must occur for at least 16 days of a 30-day period for CPT codes 99454 and 99453 to be billed. Many questioned whether this was a requirement or guidance of best practices but CMS aligns with the CPT language and is requiring that there must be 16 days of readings within a 30-day period. To confirm, this is 16 days and not 16 transmissions, meaning that multiple readings within one day will not change the requirement. This being said, for the duration of the public health emergency for COVID-19, a minimum of 2 days of data collection are required over a 30-day period, rather than the 16 days.
Many have questioned whether RPM devices must be FDA cleared as medical devices prior to use for RPM services. Per the CPT language, it is specified that devices used for RPM services must meet the FDA definition of a medical device. They also stated that they found no language requiring that these medical devices be cleared by the FDA. Devices used for RPM must have the capabilities to upload physiologic data and this requirement is not met by patient self-recorded or self-reported data. The device must digitally collect and transmit the patient’s physiologic data and must also be reasonable and necessary for the diagnosis of the patient. This information should be used to guide the development and management of the treatment plan.
This section might highlight one of the most controversial components of RPM from the CMS proposed changes for 2021. If you are currently offering RPM services, you may recall that CPT code 99457 is intended to cover the first 20 minutes of clinical staff/physician/other qualified health professional time dedicated to RPM treatment management services and that this code requires interactive communication with the patient or caregiver during the calendar month. CMS stated that 99457 and 99458 are typically furnished using communications technologies that allow for “interactive communication.” They interpret this as real-time interaction between the patient and the clinical staff member providing the service. Furthermore, they stated that this involves a minimum of a real-time, two-way audio connection that is capable of being enhanced with other kinds of data transmission, such as video connection.
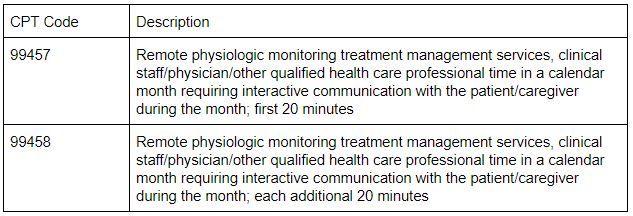
Let’s take a minute to think about what this means for the provision of RPM. For those who are currently counting clinical staff time dedicated to monitoring and analyzing incoming readings, does this meet the requirements of interactive communication? CMS states that this should not be counted towards the time for code 99457 and 99458. They interpret “interactive communication” as time spent in real-time interactive communication with the patient and/or caregiver. CMS believes that this interactive time must total at least 20 minutes within the calendar month for CPT code 99457 to be billable. The additional increments of 99458 must also be tabulated by time of interactive communication.
Telehealth services are expanding across the board, and Medicare is no exception to this rule. Certain services are proposed to be permanently added to the list of Medicare telehealth services. The Assessment of and Care Planning for Patients with Cognitive Impairment, CPT code 99483, was one of the services proposed to be added to this list. This comprehensive visit includes a cognition-focused evaluation, functional assessment, medication reconciliation, evaluation of safety, interaction with the caregiver, advance care planning, and more. While the patient’s home cannot traditionally serve as the originating site (location of the patient) for telehealth services, this restriction has been removed for the duration of the public health emergency (PHE) of COVID-19. Furthermore, it is allowable to furnish certain telehealth services via audio-only communication technology.
The Assessment of and Care Planning for Patients with Cognitive Impairment falls under direct supervision, which means that the supervising provider must be immediately available to furnish assistance, as required. For the duration of the public health emergency of COVID-19, CMS is allowing direct supervision requirements to be met through the virtual presence of the supervising provider via audio-video real-time communications technology. This is proposed to continue through the end of the calendar of the public health emergency or until December 31, 2021, whichever is later. CMS further clarified that real-time presence is not required throughout the duration of the procedure. This provides an opportunity for pharmacists to work with providers to offer telehealth services under direct supervision with flexibilities until at least December 31, 2021.
For the 2020 calendar year, CMS added G2058 to the family of billable care management codes for chronic care management (CCM). This code is billable for additional increments of 20 minutes of clinical staff time beyond the initial 20 minutes of time for non-complex CCM. CMS is now proposing the adoption of a CPT code that will replace the HCPCS code, G2058. Many commenters requested a crosswalk for this transition and CMS stated that this may become part of the review for 2022 rulemaking. Since CMS proposed to keep the same language and rules for this new CPT code, it is unlikely that this will significantly impact pharmacist-led clinical services, but this is something to keep in mind for the future. It is always important to ensure that the correct codes have been added to collaborating clinic’s fee schedules for billing purposes.
Right now, these are the proposed changes for 2021. This means that CMS is asking for input from the medical community and other members of the public on whether these proposed changes accurately describe the clinical scenarios of these services. If you are currently offering any of these services and have comments for the proposed changes, we encourage you to provide feedback to CMS. There is opportunity for growth in 2021 with the expansion of telehealth and remote services. While the PHE of COVID-19 continues to leave uncertainties of what 2021 will look like, CMS continues to look towards expanding opportunities to meet the changing needs of Medicare patients. It is likely that other payers will follow suit and we encourage you to continue to monitor the situation with local payer opportunities for pharmacist-led clinical services.
References:
Centers for Medicare & Medicaid Services. Medicare Program; CY 2021 Payment Policies under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug under a Prescription Drug Plan or an MA-PD plan; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; and Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy. Woodlawn, MD: Federal Register, 2020.

